Hutchisons symptomer forsvandt efter 12 timer. Men, siger han, 'Ingen forberedte mig på sværhedsgraden af dette.'
Han siger, at offentligheden bør være bedre forberedt, end han var, fordi en delmængde af mennesker kan have intense, omend forbigående bivirkninger, kaldet reaktogenicitet, fra Modernas vaccine. Nogle sundhedseksperter er enige.
Public needs to prep for vaccine side effects
"Somebody needs to address the elephant: What about vaccine reactogenicity? While it's ... not going to cause any long-term issues ... how is that perception going to go with the public once they start receiving it?" asks Deborah Fuller, a vaccinologist at the University of Washington, Seattle, whose lab is developing second-generation RNA vaccines against COVID-19. She worries the side effects could feed vaccine hesitancy. "I feel like it's being glossed over."Those concerns arise after a week of good news about coronavirus vaccines: Both Moderna and Pfizer, with BioNTech, announced their messenger RNA (mRNA) vaccines reached 95% efficacy in clinical trials of tens of thousands of people. The firms added that the trials showed no serious safety concerns.
Comment: Vaccines are not efficient and safe as Big Pharma likes to advertize them.
- 7 disturbing questions AstraZeneca needs to answer about its Covid-19 vaccine as it pushes for emergency authorization
- UK scientists confirm efficacy of AstraZeneca's Covid-19 vaccine, day after one volunteer reported dead in Brazil
- Whistleblowers tell the truth about vaccine safety and effectiveness
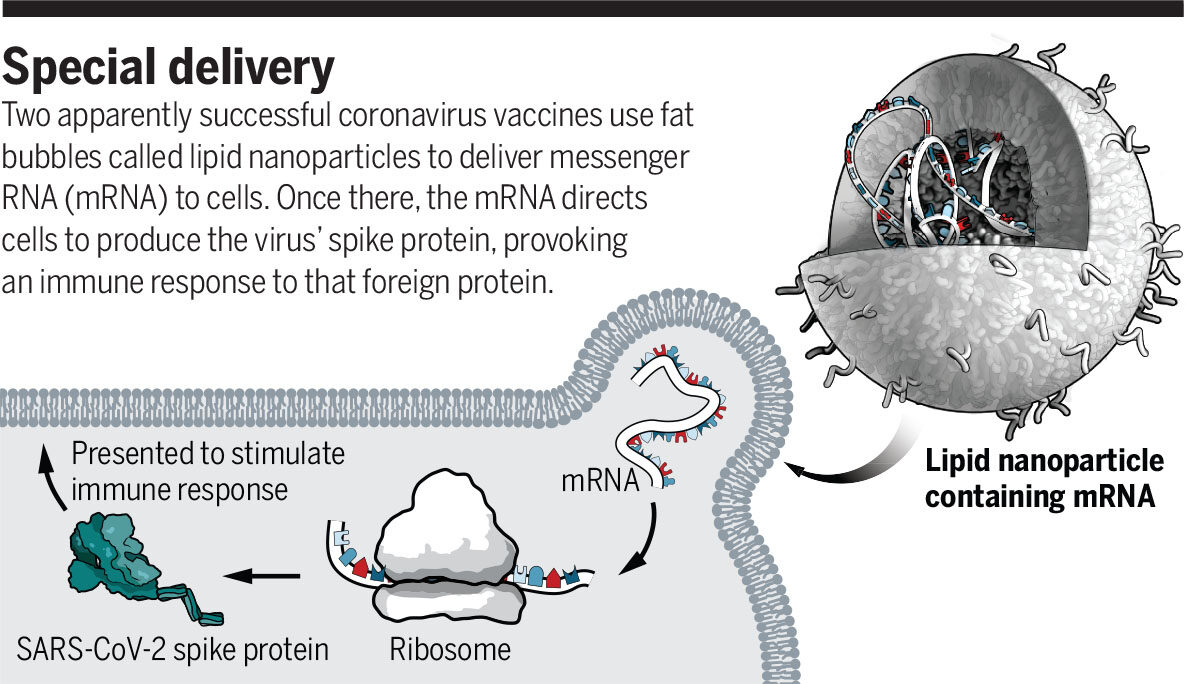
Both vaccines consist of a snippet of genetic code directing a production of the coronavirus' spike protein, delivered in a tiny fat bubble called a lipid nanoparticle. Some researchers suspect the immune system's response to that delivery vehicle is causing the short-term side effects.
Those transient reactions should not dissuade people from getting vaccinated in the face of a pandemic virus that kills at least one in 200 of those it infects, says Florian Krammer, a vaccinologist at the Icahn School of Medicine at Mount Sinai, who participated in Pfizer's trial. Sore arms, fevers, and fatigue are "unpleasant but not dangerous," he says. I'm not concerned about [reactogenicity] at all."
Comment: All available data show that the real mortality of COVID-19 is less than the seasonal flu.
- Better Flu Season Than Average? Covid-19 Yet to Impact Europe's Overall Mortality
- Study of All-cause Mortality During Covid-19: No Plague, But Likely Mass Homicide by Government Response
- Trump claims media is reporting high Covid-19 numbers to misinform people about mortality rate
- Danish study reveals that coronavirus may be almost 20x less deadly than WHO predicted
- COVID 19 is a statistical nonsense
Most people will escape "severe" side effects, defined as those that prevent daily activity. Fewer than 2% of recipients of the Pfizer and Moderna vaccines developed severe fevers of 39°C to 40°C. But if the companies win regulatory approvals, they're aiming to supply vaccine to 35 million people worldwide by the end of December. If 2% experienced severe fever, that would be 700,000 people.Other transient side effects would likely affect even more people. The independent board that conducted the interim analysis of Moderna's huge trial found that severe side effects included fatigue in 9.7% of participants, muscle pain in 8.9%, joint pain in 5.2%, and headache in 4.5%. In the Pfizer/BioNTech vaccine trial, the numbers were lower: Severe side effects included fatigue (3.8%) and headache (2%).
But that's a higher rate of severe reactions than people may be accustomed to. "This is higher reactogenicity than is ordinarily seen with most flu vaccines, even the high-dose ones," says Arnold Monto, an epidemiologist at the University of Michigan School of Public Health.
So front-line public health workers will need "to have a story that gets out in front of [stories like Hutchison's] — that responds to the way that people are going to try to make that a story about vaccine injury," says Bernice Hausman, an expert on vaccine controversy at the Pennsylvania State University College of Medicine.
Transparency is key, Hausman emphasizes. Rather than minimizing the chance of fever, for instance, vaccine administrators could alert people that they may experience a fever that can feel severe but is temporary. "That would go a significant way toward people feeling like they are being told the truth." Adds Drew Weissman, an immunologist at the University of Pennsylvania whose research contributed to both vaccines: "The companies just have to warn people: 'This is what you need to expect. Take Tylenol and suck it up for a day.'"
Comment: The truth is that these COVID-19 vaccines are much more dangerous than it is officially said.
- Disaster: 20% of Moderna's human test subjects sustained severe injuries from Gates-Fauci coronavirus vaccine
- Vaccine for the China virus — the planet is the guinea pig for a vast experiment
- CROOKS: Two Moderna executives sold $30 million of stock before truth about vaccine trial failure was uncovered
Hausman also sees a need to support people who have serious reactions. For example, people may need "a hotline with a nurse triaging ... figuring out if you need to go to the hospital or not. Will your medical expenses be covered if you do? These are important questions."
Both Moderna's and Pfizer/BioNTech's vaccines require two doses separated by several weeks. Reactogenicity is typically higher after a second dose, Weissman says. The side effects "mean the vaccine is working well. ... [It] means you had such a good immune response to the first dose and now you are seeing the effects of that," he says.
"We suspect the lipid nanoparticle causes the reactogenicity, because lipid nanoparticles without mRNA in them do the same thing in animals," Weissman says. "We see production, in the muscle, of inflammatory mediators that cause pain, [redness], swelling, fever, flulike symptoms, etc."
Hutchison hopes better vaccines are on the way. Still, he says, "Given that COVID can kill or incapacitate people, everybody should bite the bullet and expect a rough night. ... Get lots of naproxen."



Kommentar: Big Pharma is pushing dangerous drugs and vaccines on people. The "cure" is much more dangerous than the virus itself.